The date of March 11 used to be known for the Madrid train bombings of 2004. It is now known for a much greater disaster, the Fukushima nuclear disaster of 2011. I am feeling an inclination to write a series of posts on the subject.
Shortly after the Fukushima disaster, Charlie Martin wrote a post on some of the technical aspects of radioactivity. I found this post to be helpful, but I felt a need to go slower than he did.
Once upon a time, everyone knew that all atoms were stable. An atom of one kind of element never turned into an atom of some other element. A little over 100 years ago, in the normal process of scientific investigation, this was discovered to be false. Certain elements (like radium, for example) were found to be inherently unstable, changing into other elements, and emitting particles and/or energy in the process. This process is called radioactive decay.
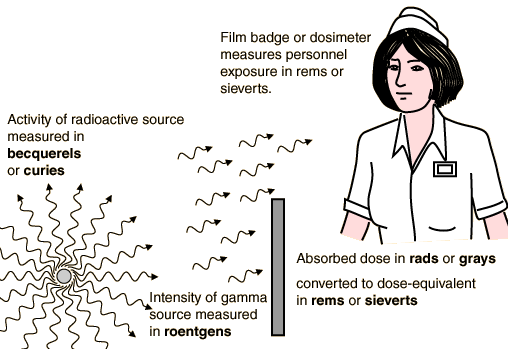
Becquerels, grays and seiverts
Because Henri Becquerel and Marie Curie were the discoverers of this phenomenon. Therefore one unit used to measure radioactivity is the becquerel (1 decay/second). Another unit is the curie (the number of decays per second in one gram of radium). This is important to know to avoid a hysterical reaction when you read a media report of a large number of becquerels associated with the Fukushima disaster. One gram of radium produces 37,000,000,000 decays per second, or 37 billion becquerels..
Furthermore, different materials have different numbers of decays per second. For example, it takes 16.3 grams of plutonium to produce the same number of decays per second as one gram of radium. It only takes 0.0001 gram of tritium (a radioactive isotope of hydrogen) to produce the same number of decays per second as one gram of radium.
Different materials release different kinds of particles and different amounts of energy when they decay. Therefore, knowing the number of becquerels of a source of radioactivity is insufficient. I found this image from a Georgia State University webpage to be helpful
A roentgen (named in honor of German physicist Wilhelm Röntgen, discoverer of X-rays) is a unit of electric charge per kilogram. Normal background radiation is about .2 roentgens per year. 500 roentgens in five hours (about 4,380,000 times the background radiation) is considered a lethal exposure.
A gray is a unit of radiation dose. A gray is defined by one joule of radiation energy absorbed by one kilogram of matter. I realize that doesn’t help very much. How much is a joule? One watt for one second. If you have a 60-watt light bulb on for 15 seconds, that’s 900 joules. Many people are charged a certain amount per kilowatt-hour for electricity. One kilowatt-hour is 3,600,000 joules. A rad is defined as .01 gray
A sievert measures the same unit as a gray, but takes into account the biological context of the matter receiving the dose, while the gray does not. Different kinds of particles and energies have significantly different amounts of damage to human tissue. Therefore, the dose in grays is multiplied by a “quality factor” or “weighing factor” to obtain a dose in sieverts. The quality factor is as low as 1 (electrons and gamma rays) and as high as 20 (alpha particles, neutrons at certain energies). A rem (roentgen equivalent in man) is defined as .01 sievert.
The scientific community is phasing out the use of curies, roentgens, rads and rems, and standardizing the use of becquerels, grays and sieverts in discussions on radioactivity and radiation doses. A Geiger counter measures a radiation dose in millionths of sieverts per hour.
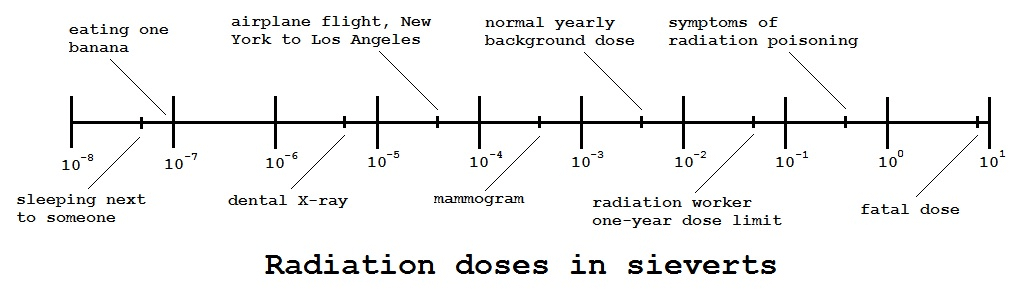
There is a certain amount of naturally occurring radioactivity in the environment, from various sources. xkcd has created a radiation dose chart which I have found helpful. I felt it might be helpful to create a simpler version of that chart:
This chart uses a logarithmic scale. For example, an airplane flight from New York to Los Angeles is roughly equivalent to 10 dental X-rays. A mammogram is roughly equivalent to 10 airplane flights, and so forth. As with roentgens, it takes millions of times the normal hourly background dose to obtain a lethal dose. The 900 joules mentioned above doesn’t seem like very much energy, but if a person weighing 100 kilograms or 220 pounds received 900 joules of radiation energy at one time, that would work out to a fatal dose of 9 sieverts, This explains why such great precautions are taken around radiation.
Someone is reading this and asking, “But how bad is it in Japan?” This photo of a Geiger counter in the city of Koriyama (40 miles away from the power plant) was recently displayed in a report in The Guardian.

I understand there are more data points than this. But people look at the picture with the Geiger counter and think “Whoa! Things must be pretty bad in Koriyama!” But the math says otherwise. All I know right now is that it doesn’t buy me anything to try and present things as either better or worse than they are. All I know right now is that doing the math and the science makes me feel stronger in the face of a disaster. There is a time for stories and feelings, and a time for becquerels, grays and sieverts.
Welcome to Mystery of Ascension! We are students and advocates of the the New Message from God. We are members of a worldwide community. We seek to assist the world in successfully navigating difficult times ahead. We seek to assist the world in successfully emerging into a greater community of intelligent life. You will also find some poetry. Find out more about us here. Contact us here.